Mural paintings
Prof. Van ‘t Hoff studies Stereochemistry
What do we see here?

This mural depicts Jacobus Henricus (Henri) van ‘t Hoff, who started the field of Stereochemistry during his PhD research in Utrecht. Stereochemistry can be described as a kind of ‘spatial chemistry’: the phenomenon that molecules that are composed of the same atoms can be structured in different ways, for example as mirror images of one another.
Different variants of the same molecule, called enantiomers, can have very different characteristics. The importance of that fact cannot be underestimated. The three-dimensional form of a molecule is one of the factors that determines its characteristics, such as its melting temperature and chemical characteristics. When applied to pharmaceuticals, the 3D shape of a molecule can make the difference between life and death: the one form of a molecule can be an effective drug, while its mirror image can be deadly. One famous example is thalidomide, a drug to treat nausea that was sold for a few years in the 1960s. The drug contained both mirror images of a molecule, but one of the two caused severe birth defects, resulting in thousands of ‘thalidomide babies’. Scientists later found that the human body can convert one mirror image into the other.
The original molecular models of Van ‘t Hoff are still on display in Rijksmuseum Boerhaave in Leiden.
 The researcher Jacobus Henricus van ‘t Hoff (1852) was an avid traveller. He studied in Delft, Leiden, Bonn and Paris, and obtained his doctorate at Utrecht University in 1874. His research into stereochemistry was so far ahead of its time that he had difficulty finding paid employment as a scientist.
The researcher Jacobus Henricus van ‘t Hoff (1852) was an avid traveller. He studied in Delft, Leiden, Bonn and Paris, and obtained his doctorate at Utrecht University in 1874. His research into stereochemistry was so far ahead of its time that he had difficulty finding paid employment as a scientist.
But his colleagues eventually began to appreciate the value of his work, and he was appointed as a Professor in Amsterdam in 1878. He received the very first Nobel Prize in Chemistry for his research into thermodynamics. By that time he was working in Berlin, where he passed away in 1911. In addition to his work in chemistry, Van ’t Hoff was a passionate lover of nature, philosophy and poetry.
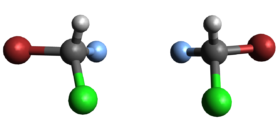
Van ’t Hoff realised that molecules have a spatial form, like marbles. See the illustration above for an example. The different colours indicate the different types of atoms: grey for carbon, white for hydrogen, blue for fluorine and red for bromide. Both molecules contain a single carbon atom, hydrogen atom, chlorine atom and bromine atom, but they are not identical because they have different spatial forms. When you look closely you can see that the molecules are mirror images of one another, like your left hand and right hand!
At first glance, that might not seem that important. But consider this: when you try to shake hands with someone, it only works when you both offer the same hand. If you offer your right hand and the other offers their left, then you can’t shake hands. The same applies to many biological molecules in your body, which need to have the precise spatial form in order to do their jobs. That makes stereochemistry a matter of life and death!